
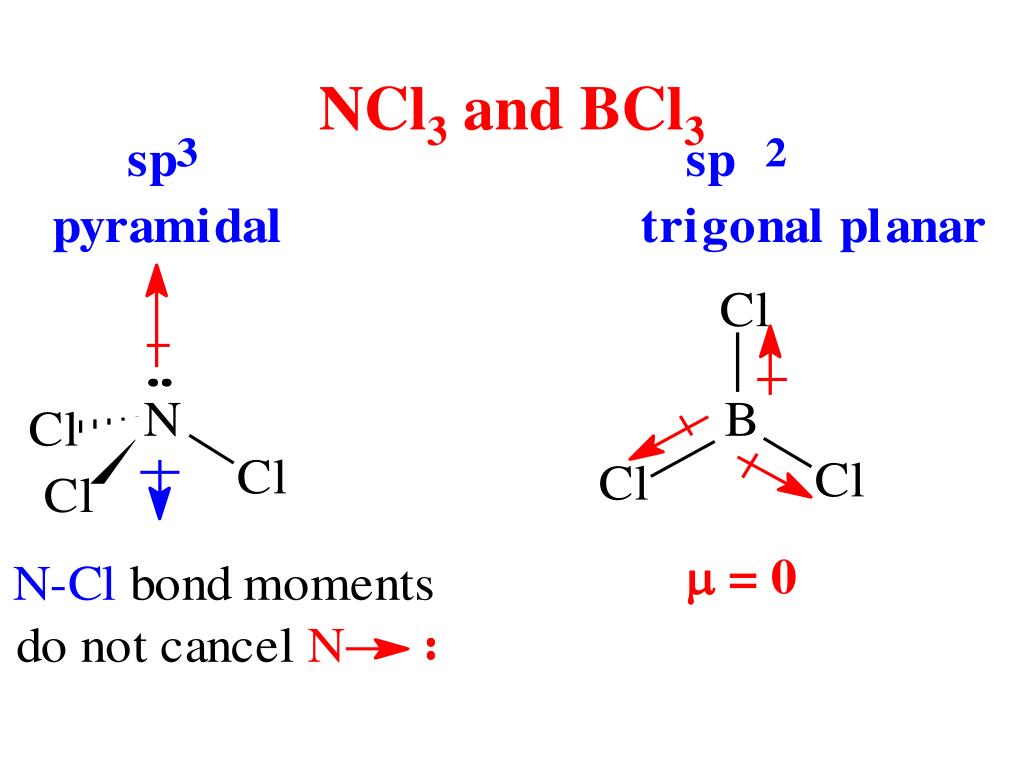
The dipole moment of boron trihydride (BH 3) is zero, whereas that of ammonia (NH 3) is 1.49D. The dipole moment of the C=O bond (2.3D) on one side of the molecule is negated by that on the opposite side of the molecule due to the linear structure of the molecule, resulting in net zero dipole moment.
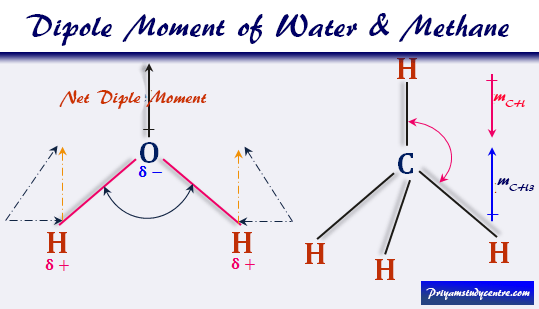
The dipole moment of the triatomic CO 2 (carbon dioxide) molecule is zero.
The dipole moment of the HCl molecule in the diatomic molecule is the same as the dipole moment of the HCl link, which is 1.03D.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 2 Structure of Atom. NCERT solutions for Class 11 Chemistry Chapter 2 Structure of Atom. NCERT notes Class 11 Chemistry Chapter 2 Structure of Atom. The bond dipole moment in chemistry can be used to depict the movement of electrons. When two atoms with different electronegativities collide, the electrons tend to migrate away from their original locations in order to approach the more electronegative atom. The arrows used to illustrate dipole moments in chemistry start at the positive charge and stop at the negative charge. The bond dipole moment (µ) is a vector quantity with the same direction as the bond axis. ? → magnitude of the partial charges ? + and ? –, In a chemical bond between two atoms with differing electronegativities, the bond dipole moment formula can be written as, Where, C stands for Coulomb and m for metre. It is measured in Debye units, which are represented by the letter ‘D.' Mathematically, dipole moment formula can be written as,ĭipole Moment=ChargeQ*Distance of separation(d) By the dipole moment definition, the dipole moment formula is the product of magnitude of charge and the distance between the positive and negative charge centres.







 0 kommentar(er)
0 kommentar(er)
